Mutants are cool, right? X-Men, Teenage Mutant Ninja Turtles and superheroes across comic books and films wow us with the special powers that have been derived from their genetic mutations. However, those fictionalgenetic mutationsare kind of hard to come by — you have to have been bitten by some special spider or exposed to some radioactive substance.
But what if doing genetic modifications was not just easy, but fast and cheap, too? Would you willingly become a mutant? Well, with a technology called CRISPR, you may be able to. Don't get us wrong — CRISPR isn't going to turn you into a superhero, but this scientific discovery has the potential to impact us in a big way.
Advertisement
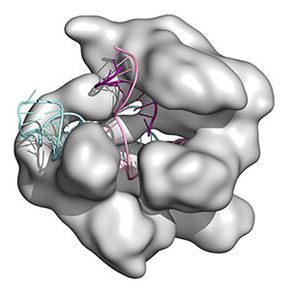
的缩写clustered regularly interspaced short palindromic repeats, CRISPR makes it possible for us to move genes from any living thing into another one, altering DNA for generations to come. It allows us to cut away genes that are doing terrible things — like those that cause disease — and replace them with segments of DNA that are innocuous.
At the same time, CRISPR technology is so powerful that humans could start to use it for more than just curing people of disease. Maybe to create more disease-resistant crops and livestock. Or to make yeast mutants that produce fuels that we can use to power our cars. We might start to get really creative and makedesigner babies, or even use the technology for evil — engineering bioweapons that are species-specific and wiping entire species off the face of the planet.
As we learn more about what theMIT Technology Review called"the biggest biotech discovery of the century," we also need to think about when we should use CRISPR and how it should be regulated.
Advertisement